Flow batteries are considered prime candidates for grid scale energy storage. In a flow battery, two liquids — one having a positive electrical charge and another having a negative electrical charge — are separated by a membrane that allows electrons to pass between both fluids while keeping them physically separate.
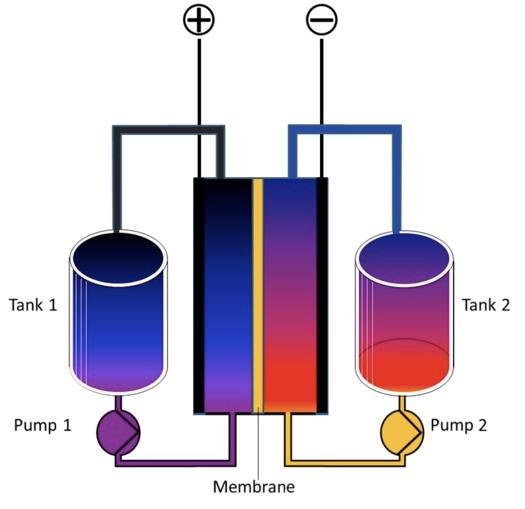 Flow batteries tend to be larger in size than comparable lithium ion batteries, which makes them problematic for use in vehicles, but space considerations are less important for batteries designed to store electricity for the grid.
Flow batteries tend to be larger in size than comparable lithium ion batteries, which makes them problematic for use in vehicles, but space considerations are less important for batteries designed to store electricity for the grid.
Until now, flow batteries have had several limitations that kept them from commercial viability. They had low energy density, required temperatures as high as 400º F to operate, and/or used toxic substances that were dangerous to the environment and cost a lot of money.
 But a team at Stanford led by William Chueh, an assistant professor of materials science and engineering, says they have solved one third of the flow battery puzzle.
But a team at Stanford led by William Chueh, an assistant professor of materials science and engineering, says they have solved one third of the flow battery puzzle.
The team has developed a liquid metal solution made from sodium and potassium — both of which are non-toxic, abundant and inexpensive — that acts at the anode for a flow battery.
The best part is, the liquid metal solution is effective at room temperature. Theoretically, this liquid metal has at least 10 times the available energy per gram and has a useful life of several thousand hours.
A new battery technology has so many different performance metrics to meet — cost, efficiency, size, lifetime, safety, etc. We can all jump up and down and wave banners proclaiming that the Earth needs renewable energy to survive, but people being what they are, only take action when the financials are favorable.
Reference- Cleantechnica, Energy Storage Report, Slac.Stanford.edu






