The new approach, according to scientists at Stanford University and the Department of Energy’s SLAC National Accelerator Laboratory, is the re-engineering of one of the heaviest components in batteries: sheets of copper or aluminum foil that are used to collect currents.
The current collector has always been considered dead weight, and until now it hasn’t been successfully exploited to increase battery performance.
But now the Stanford researchers have made the collector 80% lighter and have increased the energy density of lithium-ion batteries — how much energy they can store in a given weight — by 16–26%.
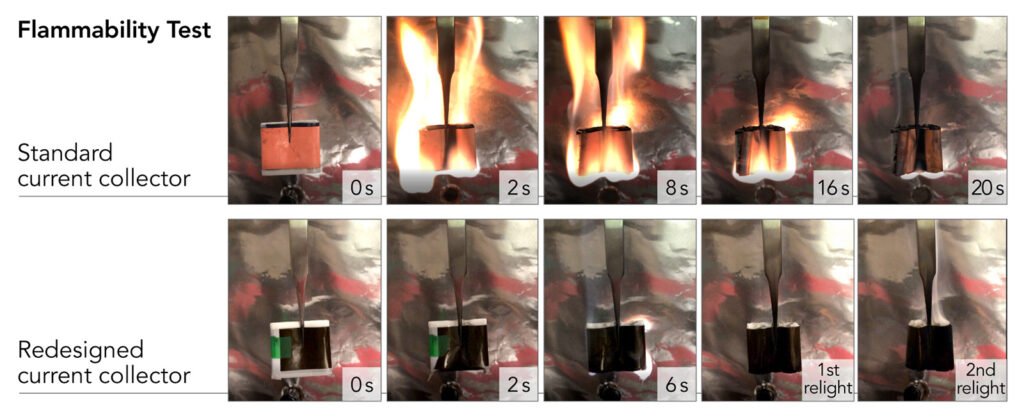
These have been redesigned so that they weigh 80% less and make the batteries fireproof.
They used a lightweight polymer known as polyimide. Polyimide resists fire and is able to take on the high temperatures created by fast battery charging.
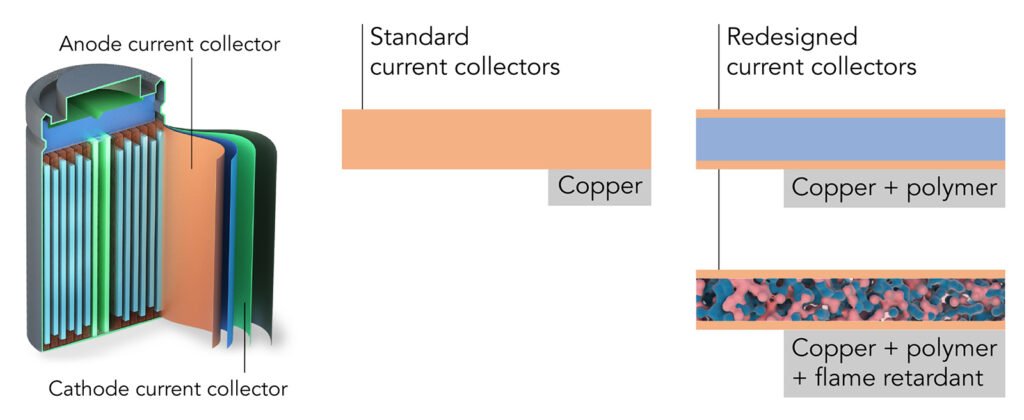
A fire retardant, triphenyl phosphate (TPP), was embedded in the polymer and then coated on both surfaces with an ultrathin layer of copper.
That’s a big jump compared to the average 3% increase achieved in recent years.
In either cylinder form or pouches, lithium-ion batteries have two current collectors for each electrode. These distribute the current that flows in or out of the electrode and account for 15% to 50% of the weight of some high-power or ultrathin batteries.

Shedding some of the battery’s weight would enable lighter devices while reducing the amount of weight EVs have to carry around.
Doing this would also enable the battery to store more energy per given weight and allow for both EVs and devices to go longer between charges.
Another benefit of reducing the weight and the flammability would have a big impact on recycling. This would make the transportation of recycled batteries less expensive.