Lithium, a vital element for clean energy technologies, faces a growing supply chain challenge. From electric vehicles to medical devices, lithium-ion batteries power our world. As demand surges, researchers are exploring innovative ways to extract this critical resource.

Traditionally, lithium comes from two sources: brines (salty underground water) and hard-rock minerals like spodumene. Brine extraction, the current leader in the U.S., relies on solar evaporation of brines in large ponds – a cost-effective but slow process (12-24 months). Conversely, hard-rock extraction, while faster, uses significant energy and creates hazardous waste.
Scientists at the Critical Materials Innovation Hub are pioneering a game-changer: mechanochemical extraction of lithium at low temperatures (MELLT). This method leverages mechanochemistry, a field that uses mechanical force to trigger chemical reactions.
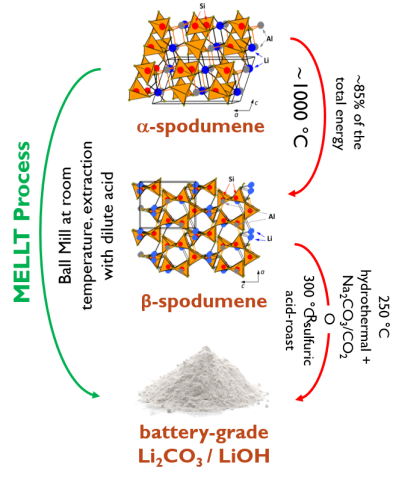
Imagine a high-energy shake-up! MELLT utilizes ball milling, where spodumene chunks and a reactant like sodium carbonate (Na2CO3) are placed in a chamber with steel balls. The chamber’s movement creates intense shear and impact, forcing the materials to react and form water-soluble lithium compounds. A simple water wash then extracts the lithium.
MELLT boasts significant advantages. It requires far less energy than traditional methods, eliminating toxic waste streams in the process. Additionally, MELLT’s speed surpasses brine extraction by a significant margin.

“Mechanochemistry offers a more sustainable and environmentally friendly approach,” explains a CMI Hub researcher. This innovation holds immense promise for a cleaner, more efficient lithium supply chain, crucial for powering a sustainable future.
Reference- Clean Technica, Futurism, Interesting Engineering, The Verge, Vox





