In a new study, researchers at Texas A&M University have described their novel plant-based energy storage device that could charge even electric cars within a few minutes in the near future.
Integrating biomaterials into energy storage devices has been tricky because it is difficult to control their resulting electrical properties, which then gravely affects the devices’ life cycle and performance.
Also, the process of making biomaterials generally includes chemical treatments that are hazardous; but this is a thing of past…

Researchers at Texas A&M University claim that they have designed an environmentally friendly energy storage device that has superior electrical performance and can be manufactured easily, safely and at much lower cost.
Energy storage devices are generally in the form of either batteries or supercapacitors.
Supercapacitors have an internal architecture that is more in line with basic capacitors. Both these devices store charge on metal plates or electrodes.
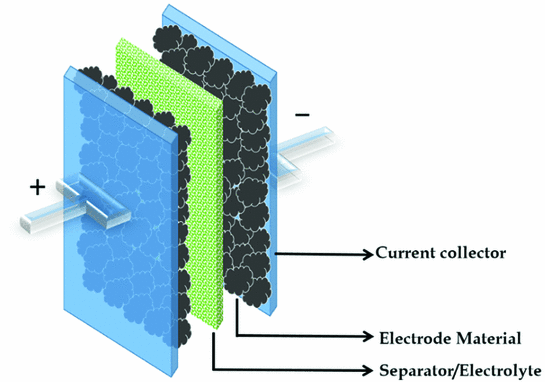
However, unlike basic capacitors, supercapacitors can be made in different sizes, shapes and designs, depending on the intended application. Furthermore, supercapacitor electrodes can also be built with different materials.
For their work, the team were attracted to manganese dioxide nanoparticles for designing one of the two supercapacitor electrodes.
To create their electrode and address the above issue the team treated purified lignin with a commonly available disinfectant called potassium permanganate.

They then applied high heat and pressure to initiate an oxidation reaction that results in the breaking down of potassium permanganate and the deposition of manganese dioxide on lignin.
Next, they coated the lignin and manganese dioxide mixture on an aluminum plate to form the green electrode.
Finally, the researchers assembled the supercapacitor by sandwiching a gel electrolyte between the lignin-manganese dioxide-aluminum electrode and another electrode made of aluminum and activated charcoal.

Upon testing their newly designed green electrode, they found that their supercapacitor had very stable electrochemical properties.
In particular, the specific capacitance, or the ability of the device to store an electrical charge, changed little, even after thousands of cycles of charging and discharging.
Also, for an optimal lignin-manganese dioxide ratio, the specific capacitance was observed to be up to 900 times more than what has been reported for other supercapacitors.
Reference- Texas A&M University Research, Journal -Energy Storage, InsideEVs






